- Lymphoid neoplasms are the top 4th and 5th cancers affecting men and women respectively in Singapore from 2017-20211. Amongst these, diffuse large B-cell lymphoma (DLBCL) is the most common type of B-cell lymphoma. The standard treatment of DLBCL in 2021 remains chemo-immunotherapy with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) but up to 45-50% of patients will relapse2.
- At present, all FDA-approved anti-CD19 CAR T-cell therapies are developed using a single-chain variable fragment (scFv) derived from murine FMC63. However, the presence of non-human (murine) scFv may induce human anti-mouse antibody (HAMA) response3 which would subsequently affect the persistency of CAR T cells in vivo1. Patients with relapsed or refractory acute lymphoblastic leukemia that had failed FMC63-derived CD19 CAR Lv is responsive to humanized CD19 CAR Lv (Clinical Trials # NCT02374333 & ChiCTR1900024456)
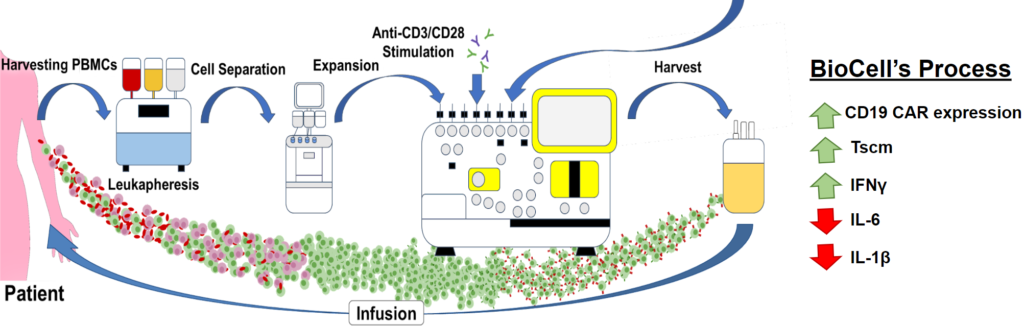
- Chimeric antigen receptor (CAR) -modified T-cell therapy against CD19 has emerged as a significant therapy against relapsed/refractory lymphomas with an overall response rate ranging from 53% to 83%4. However, patients often require a high dosage of T-cell infusion leading to increased demand for lentiviral vectors to overcome the challenge of transducing primary T cells.
- BioCell’s in-house CD19 CAR Lv is constructed using a humanized CD19 CAR T design. Furthermore, we have recently identified a novel soluble transduction enhancer that exhibits similar T-cell transduction efficiencies as mediated by RetroNectin which is cumbersome to use due to the requirement to pre-coat culture dishes or bags before cell seeding. More importantly, CAR-T products prepared in the presence of this soluble transduction enhancer exhibit markedly downregulates cytokines (e.g., IL-6) associated with cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome, without affecting lymphoma cell kill, which indicate improved patient safety and treatment outcomes.
References
- https://www.singaporecancersociety.org.sg/learn-about-cancer/cancer-basics/common-types-of-cancer-in-singapore.html
- Susanibar‐Adaniya, S., & Barta, S. K. (2021). 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. American journal of hematology, 96(5), 617-629.
- Blanco, I., Kawatsu, R et al., (1997). Anti-idiotypic response against murine monoclonal antibodies reactive with tumor-associated antigen TAG-72 Journal of clinical immunology, 17, 96-106.
- Yamauchi, N., & Maruyama, D. (2024). Current development of chimeric antigen receptor T‐cell therapy for diffuse large B‐cell lymphoma and high‐grade B‐cell lymphoma. European Journal of Haematology.

